NCERT Exemplar Class 11 Biology Chapter 9 Biomolecules are part of NCERT Exemplar Class 11 Biology. Here we have given NCERT Exemplar Class 11 Biology Chapter 9 Biomolecules.
NCERT Exemplar Class 11 Biology Chapter 9 Biomolecules
Multiple Choice Questions
Q1. It is said that elemental composition of living organisms and that of inanimate objects (like earth’s crust) are similar in the sense that all the major elements are present in both. Then what would be the difference between these two . groups? Choose a correct answer from the following.
(a) Living organisms have more gold in them than inanimate objects
(b) Living organisms have more water in their body than inanimate objects
(c) Living organisms have more carbon, oxygen and hydrogen per unit mass than inanimate objects
(d) Living organisms have more calcium in them than inanimate objects.
Ans: (c)
| Element | % Weight of | |
| Earth’s Crust | Human Body | |
| Hydrogen (H) | 0.14 | 0.5 |
| Carbon (C) | 0.03 | 18.5 |
| Oxygen(0) | 46.6 | 65.0 |
| Nitrogen (N) | very little | 3.3 |
| Sulphur (S) | 0.03 | 0.3 |
| Sodium (Na) | 2.8 | 0.2 |
| Calcium (Ca) | 3.6 | 1.5 |
| Magnesium (Mg) | 2.1 | 0.1 |
| Silicon (Si) | 27.7 | negligible |
Q2. Many elements are found in living organisms either free or in the form of compounds. One of the following is not found in living organisms.
(a) Silicon (b) Magnesium (c) Iron (d) Sodium
Ans: (a) See Answer 2.
Q3. Aminoacids, as the name suggests, have both an amino group and a carboxyl group in their structure. In addition, all naturally occurring aminoacids (those which are found in proteins) are called L-aminoacids. From this, can you guess from which compound can the simplest aminoacid be made?
(a) Formic acid (b) Methane (c) Phenol acid (d) Glycine
Ans: (d) Glycine is an amino acid (which have both an amino group and a carboxyl group in their structure).
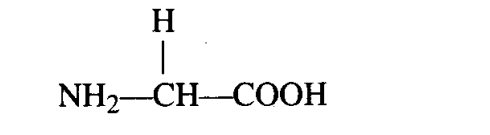
Q4. Many organic substances are negatively charged, e.g., acetic acid, while others are positively charged e.g., ammonium ion. An aminoacid under certain conditions would have both positive and negative charges simultaneously in the same molecule. Such a form of aminoacid is called
(a) Positively charged form (b) Negatively charged form
(c) Neutral form (d) Zwitterionic form
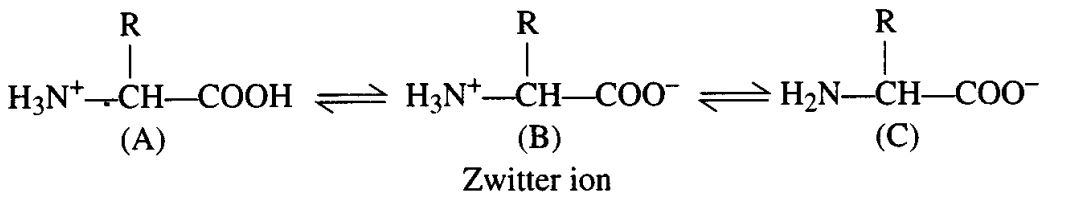
Ans: (d) In aqueous solution, the carboxyl group can lose a proton and amino group can accept a proton, giving rise to a dipolar ion called Zwitter ion. Zwitter ion is neutral but contains both positive and negative charges.
Q5. Sugars are technically called carbohydrates, referring to the fact that their formulae are only multiple of C(H20). Hexoses therefore have six carbons, twelve hydrogens and six oxygen atoms. Glucose is a hexose. Choose from among the following another hexose.
(a) Fructose (b) Erythrose ~(c) Ribulose (d) Ribose
Ans: (a) Sugars are technically called carbohydrates, referring to the fact that their formulae are only multiple of C(H20). Hexoses therefore have six carbons, twelve hydrogens and six oxygen atoms. E.g., glucose and fructose.
Q6. When you take cells or tissue pieces and grind them with an acid in a mortar and pestle, all the small biomolecules dissolve in the acid. Proteins polysaccharides and nucleic acids are insoluble in mineral acid and get precipitated. The acid soluble compounds include amino acids, nucleosides, small sugars etc. When one adds a phosphate group to a nucleoside one gets another acid soluble biomolecule called
(a) Nitrogen base
(b) Adenine
(c) Sugar phosphate
(d) Nucleotide
Ans: (d) Neucliotide = base + sugar + phosphate
Q7. When we homogenise any tissue in an acid, the acid soluble pool represents
(a) Cytoplasm (b) Cell membrane
(c) Nucleus (d) Mitochondria
Ans: (a) When we homogenise any tissue in an acid, the acid soluble pool represents cytoplasm.
Q8. The most abundant chemical in living organisms could be
(a) Protein (b) Water (c) Sugar (d) Nucleic acid
Ans: (b) Most abundant component of cell is water.
| Component | % of the total Cellular Mass |
| Water | 70-90 |
| Proteins | 10-15 |
| Nucleic acids | 5-7 |
| Carbohydrates | 3 |
| Lipids | 2 |
| Ions | 1 |
Q9. A homopolymer has only one type of building block called monomer repeated V number of times. A heteropolymer has more than one type of monomer. Proteins are heteropolymers usually made of aminoacids. While a nucleic acid like DNA or RNA is made up of only 4 types of nucleotide monomers, proteins are made of
(a) 20 types of monomers (b) 40 types of monomers
(c) 30 types of monomers (d) only one type of monomer
Ans: (a) A homopolymer has only one type of building block called monomer repeated V number of times. A heteropolymer has more than one type of monomer. Proteins are heteropolymers usually made of amino acids. While a nucleic acid like DNA or RNA is made of of only 4 types of nucleotide monomers, proteins are made of 20 types of monomers.
Q10. Proteins perform many physiological functions. For example, some proteins function as enzymes. One of the following represents an additional function that some proteins discharge
(a) Antibiotics
(b) Pigment conferring colour to skin
(c) Pigment making colours of flowers
(d) Hormones
Ans: (d) Proteins perform many physiological functions. For example, some proteins function as enzymes. Hormones represents an additional function that some proteins discharge (like insulin).
Q11. Glycogen is a homopolymer made of
(a) Glucose units (b) Galactose units
(c) Ribose units (d) Amino acids
Ans: (a) Glycogen is a homopolymer made of glucose units.
Q12. The number of ‘ends’ in a glycogen molecule would be
(a) Equal to the number of branches plus one
(b) Equal to the number of branch points
(c) One ‘
(d) Two, one on the left side and another on the right side
Ans: (d) In a polysaccharide chain (say glycogen), the right end is called the reducing end and the left end is called the non-reducing end.
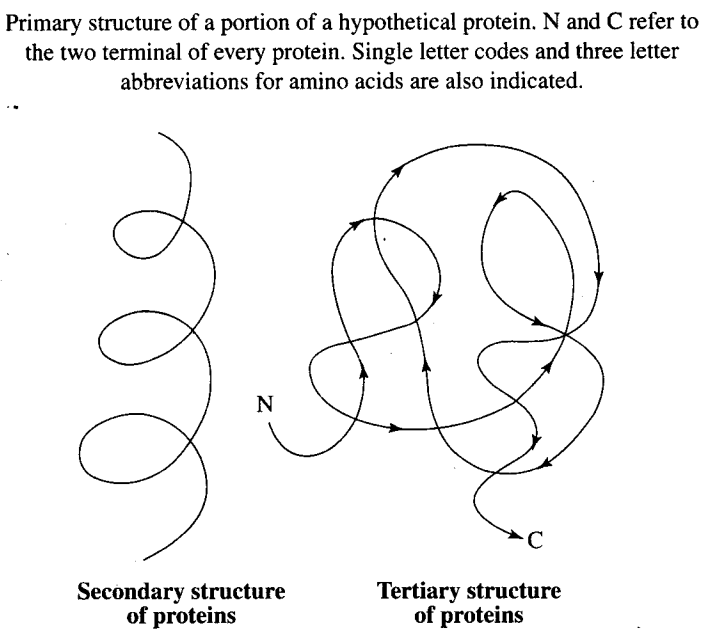
Q13. The primary structure of a protein molecule has
(a) Two ends (b) One end (c) Three ends (d) No ends
Ans: (a) The primary structure of a protein molecule has two ends.
A protein is imagined as a line, the left end represented by the first amino acid and the right end is represented by the last amino acid. The first amino acid is also called as N-terminal amino acid. The last amino acid is called the C-terminal amino acid.
Q14. Enzymes are biocatalysts. They catalyse biochemical reactions. In general they reduce activation energy of reactions. Many physico-chemical processes are enzyme mediated.’ Some examples of enzyme mediated reactions are given below. Tick the wrong entry.
(a) Dissolving C02 in water
(b) Unwinding the two strands of DNA .
(c) Hydrolysis of sucrose
(d) Formation of peptide bond
Ans: (a) Dissolving C02 in water is a physical process.
Very Short Answer Type Questions
Q1. Medicines are either man made (i.e., synthetic) or obtained from living organisms like plants, bacteria, animals etc. and hence the latter are called natural products. Sometimes natural products are chemically altered by man to reduce toxicity or side effects. Write against each of the following whether they were initially obtained as a natural product or as a synthetic chemical.
a. Penicillin
b. Sulfonamide
c. Vitamin C
d. Growth Hormone
Ans: a. Penicillin: Natural product
b. Sulfonamide: Synthetic chemical
c. Vitamin C: Natural product
d. Growth Hormone: Natural product
Q2. Select an appropriate chemical bond among ester bond, glycosidic bond, peptide bond and hydrogen bond and write against each of the following.
a. Polysaccharide
b. Protein
c. Fat
d. Water
Ans: a. Polysaccharide: Glycosidic bond
b. Protein: Peptide bond
c. Fat: Ester bond
d. Water: Hydrogen bond
Q3. Write the name of any one aminoacid, sugar, nucleotide and fatty acid.
Ans: Glycine (amino acid), Ribose (sugar), Cytidylic acid (nucleotide) and
Arachidonic acid (fatty acid).
Q4. Reaction given below is catalysed by oxidoreductase between two substrates A and A’, complete the reaction. A reduced + A‘ oxidised -»
Ans: A reduced + A’ oxidised —»A oxidised + A’ reduced
Q5. How are prosthetic groups different from co-factors?
Ans: Prosthetic groups are organic compounds and are distinguished from other cofactors in that they are tightly bound to the apoenzyme. For example, in peroxidase and catalase, which catalyze the breakdown of hydrogen peroxide to water and oxygen, haem is the prosthetic group and it is a part of the active site of the enzyme.
Cofactor may be organic or inorganic (metal ions).
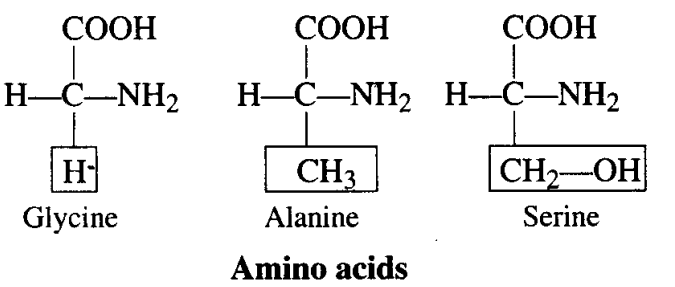
Q6. Glycine and Alanine are different with respect to one substituent on the a-carbon. What are the other common substituent groups?
Ans: The R-group in these proteinaceous amino acids could be a hydrogen (the amino acid is called glycine), a methyl group (alanine), hydroxy methyl (serine), etc.
Q7. Starch, Cellulose, Glycogen, Chitin are polysaccharides found among the following. Choose the one appropriate and write against each.
Cotton fibre __________
Exoskeleton of Cockroach __________
Liver __________
Peeled potato __________
Ans: Cotton fibre : Cellulose
Exoskeleton of Cockroach : Chitin
Liver: Glycogen
Peeled potato: Starch
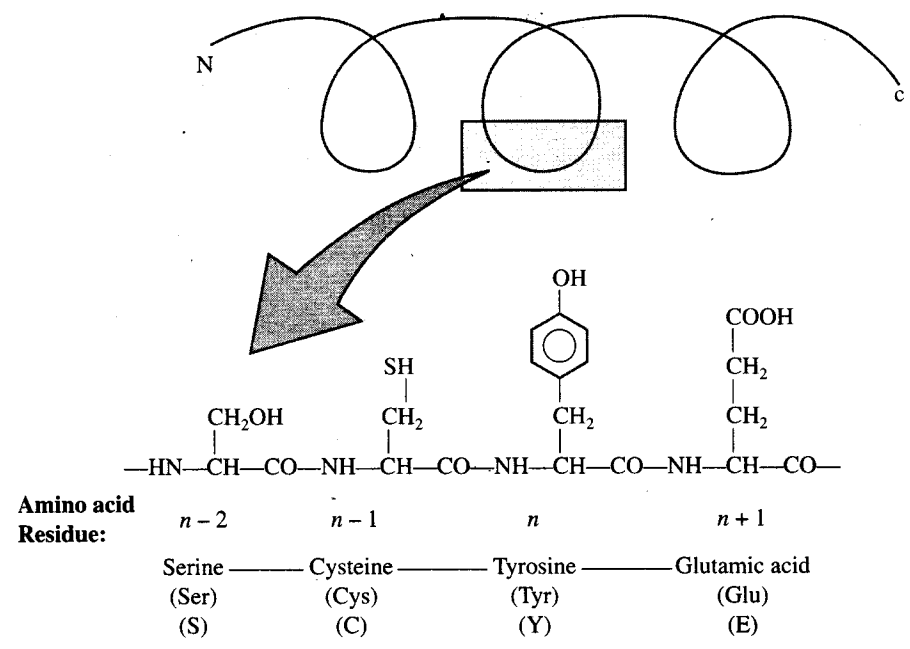
Q4. Nucleic acids exhibit secondary structure, justify with example.
Ans: Nucleic acids exhibit a wide variety of secondary structures. For example, one of the secondary structures exhibited by DNA is the famous Watson—Crick model. This model says that DNA exists as a double helix. The two strands of polynucleotides are antiparallel, i.e. run in the opposite direction. The backbone is formed by the sugar-phosphate-sugar chain. The nitrogen bases are projected more or less perpendicular to this backbone but face inside. A and G of one strand compulsorily base pairs with T and C, respectively, on the other strand. There are two hydrogen bonds between A and T and three hydrogen bonds between G and C. Each strand appears like a helical staircase. Each step of ascent is represented by a pair of bases. At each step of ascent, the strand turns 36°. One full turn of the helical strand would involve ten steps or ten base pairs. Attempt drawing a line diagram. The pitch would be 34 A. The rise per base pair would be 3.4 A. This form of DNA with the above mentioned salient features is called B-DNA.
Q5. Comment on the statement “living state is a non-equilibrium steady state to be able to perform work”.
Ans: The most important fact of biological systems is that all living organisms exist in a steady-state characterised by concentrations of each of these biomolecules. These biomolecules are in a metabolic flux. Any chemical or physical process moves spontaneously to equilibrium. The steady state is a non-equilibrium state. One should remember from physics that systems at equilibrium cannot perform work. As living organisms work continuously, they cannot afford to reach equilibrium. Hence the living state is a non-equilibrium steady-state to be able to perform work; living process is a constant effort to prevent falling into equilibrium. This is achieved by energy input. Metabolism provides a mechanism for the production of energy. Hence the living state and metabolism are synonymous. Without metabolism there cannot be a living state.
Long Answer Type Questions
Q1. Formation of enzyme-substrate complex (ES) is the first step in catalysed reactions. Describe the other steps till the formation of product.
Ans: The catalytic cycle of an enzyme action can be described in the following steps:
(1) First, the substrate binds to the active site of the enzyme, fitting into the active site.
(2) The binding of the substrate induces the enzyme to alter its shape, fitting more tightly around the substrate.
(3) The active site of the enzyme, now in close proximity of the substrate breaks the chemical bonds of the substrate and the new enzyme-product complex is formed.
(4) The enzyme releases the products of the reaction and the free enzyme is ready to bind to another molecule of the substrate and run through the catalytic cycle once again.
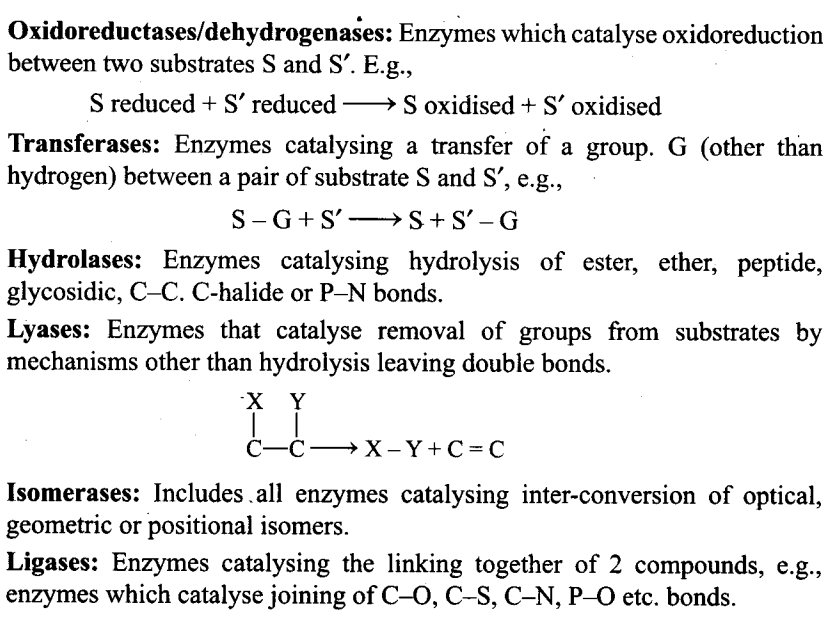
Q2. What are different classes of enzymes? Explain any two with the type of reaction they catalyse.
Ans: Enzymes are divided into 6 classes each with 4—13 subclasses and named accordingly by a four-digit number.
Q3. Nucleic acids exhibit secondary structure. Describe through Watson-Crick Model.
Ans: Nucleic acids exhibit a wide .variety of secondary structures. For example, one of the secondary structures exhibited by DNA is the famous Watson- Crick model. This model says that DNA exists as a double helix. The two strands of polynucleotides are antiparallel, i.e. run in the opposite direction. The backbone is formed by the sugar—phosphate—sugar chain. The nitrogen bases are projected more or less perpendicular to this backbone but face inside. A and G of one strand compulsorily base pairs with T and C, respectively, on the other strand. There are two hydrogen bonds between A
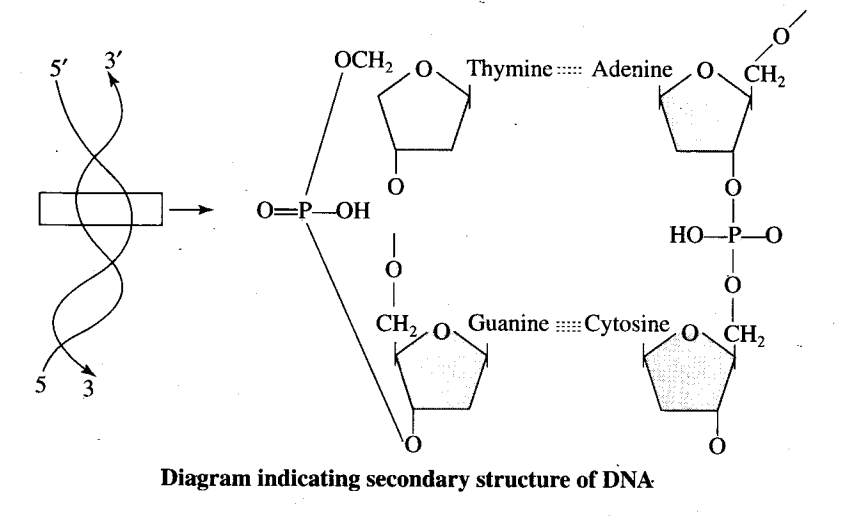
and T and three hydrogen bonds between G and C. Each strand appears like a helical staircase. Each step of ascent is represented by a pair of bases. At each step of ascent, the strand turns 36°. One full turn of the helical strand would involve ten steps or ten base pairs. Attempt drawing a line diagram. The pitch would be 34 A. The rise per base pair would be 3.4 A. This form of DNA with the above mentioned salient features is called B-DNA.
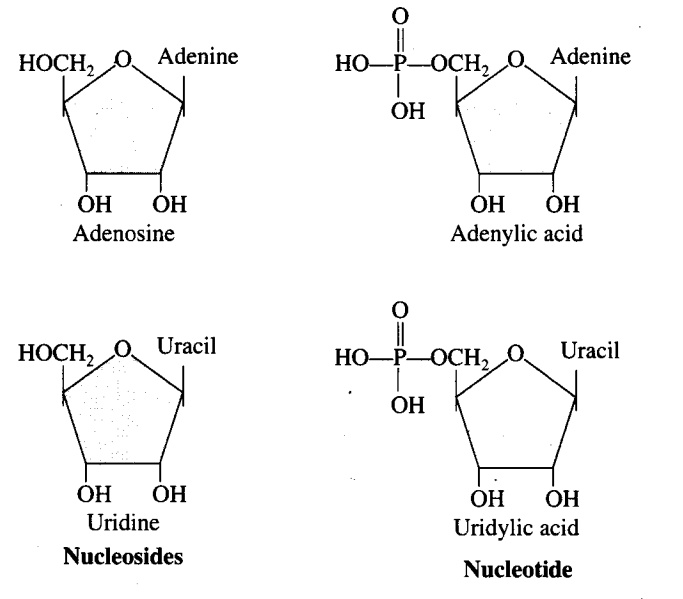
Q4. What is the difference between a nucleotide and nucleoside? Give two examples of each with their structure.
Ans: Living organisms have a number of carbon compounds in which heterocyclic rings can be found. Some of these are nitrogen bases—adenine, guanine, cytosine, uracil and thymine. When found attached to a sugar, they are called nucleosides. If a phosphate group is also found esterified to the sugar they are called nucleotides. Adenosine, guanosine, thymidine, uridine and cytidine are nucleosides. Adenylic acid, thymidylic acid, guanylic acid, uridylic acid and cytidylic acid are nucleotides.
Q5. Describe various forms of lipid with a few examples.
Ans: Lipids are generally water insoluble. They could be simple fatty acids. A fatty acid has a carboxyl group attached to an R-group. The R-group could be a methyl (-CH3), or ethyl (—C2H5) or higher number of-CH2 groups (1 carbon to 19 carbons). For example, palmitic acid has 16 carbons including carboxyl carbon. Arachidonic acid has 20 carbon atoms including the carboxyl carbon. Fatty acids could be saturated (without double bond) or unsaturated (with one or more C=C double bonds). Another simple lipid is glycerol which is trihydroxy propane.
• Many lipids have both glycerol and fatty acids. Here the fatty acids are found esterified with glycerol. They can be then monoglycerides,
diglycerides and triglycerides. These are also called fats and oils based on melting point. Oils have lower melting point (e.g., gingely oil) and hence remain as oil in winters.
• Some lipids have phosphorous and a phosphorylated organic compound in them. These are phospholipids. They are found in cell membrane. Lecithin is one example. Some tissues especially the neural tissues have lipids with more complex structures.
NCERT Exemplar Class 11 Biology Solutions
- Chapter 1 The Living World
- Chapter 2 Biological Classification
- Chapter 3 Plant Kingdom
- Chapter 4 Animal Kingdom
- Chapter 5 Morphology of Flowering Plants
- Chapter 6 Anatomy of Flowering Plants
- Chapter 7 Structural Organisation in Animals
- Chapter 8 Cell: The Unit of Life
- Chapter 9 Biomolecules
- Chapter 10 Cell Cycle and Cell Division
- Chapter 11 Transport in Plants
- Chapter 12 Mineral Nutrition
- Chapter 13 Photosynthesis in Higher Plants
- Chapter 14 Respiration in Plants
- Chapter 15 Plant Growth and Development
- Chapter 16 Digestion and Absorption
- Chapter 17 Breathing and Exchange of Gases
- Chapter 18 Body Fluids and Circulation
- Chapter 19 Excretory Products and Their Elimination
- Chapter 20 Locomotion and Movement
- Chapter 21 Neural Control and Coordination
- Chapter 22 Chemical Coordination and Integration
NCERT Exemplar ProblemsMathsPhysicsChemistryBiology
We hope the NCERT Exemplar Class 11 Biology Chapter 9 Biomolecules help you. If you have any query regarding NCERT Solutions for NCERT Exemplar Class 11 Biology Chapter 9 Biomolecules, drop a comment below and we will get back to you at the earliest.
<!–
–>