To Study the Variation of Cell Potential in Zn | Zn2+ || Cu2+ | Cu Cell with Change in Concentration of Electrolytes (CuS04 and ZnS04) at Room Temperature
Chemistry Lab ManualNCERT Solutions Class 12 Chemistry Sample Papers
Theory
Reduction potential of an electrode increases with increase in concentration of the electrolyte.
Mn+(aq) + ne– ———-> M(s)
In the zinc-copper electrochemical cell zinc electrode acts as anode while copper electrode acts as cathode.
Apparatus and Chemicals
One beaker, a porous pot, connecting wires, milli voltmeter, sand paper, zinc strip, copper strip, 1 M ZnS04 solution and 1 M CuS04 solution.
Procedure
- Take copper sulphate solution in a clean beaker.
- Clean the copper strip with the help of sand paper and dip it into copper sulphate solution.
- Take zinc sulphate solution in a porous pot.
- Clean the zinc strip with the help of sand paper and dip it into zinc sulphate solution.
- Keep the porous pot in the beaker.
- Connect the copper strip with the positive terminal and zinc strip with the negative terminal of a voltmeter as shown in Fig.
- Note the position of the pointer in the voltmeter and record the reading in your notebook.
Repeat the experiment by taking different concentrations of zinc sulphate and copper sulphate solutions.
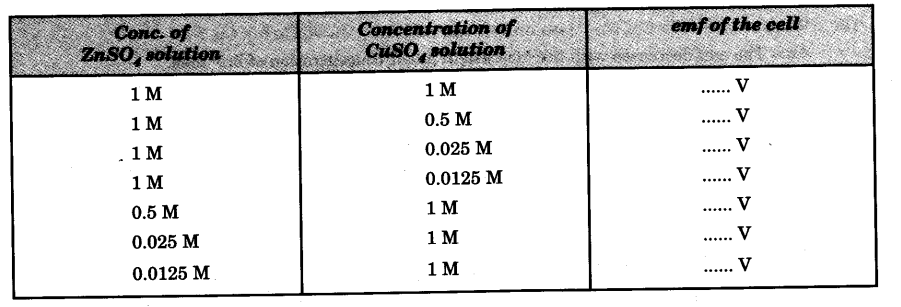
Observation
Conclusion
EMF of the cell increases with decrease in cone, of the electrolyte around anode and increase in cone, of the electrolyte around cathode.
<!–
–>