To Study the Reaction Rate of the Reaction Between Potassium Iodate (KIO3) and Sodium Sulphite (Na2S03) Using Starch Solution as Indicator
Chemistry Lab ManualNCERT Solutions Class 12 Chemistry Sample Papers
Theory
In acidic medium, potassium iodate is reduced to iodide by sulphite. The reaction takes place through the following steps :
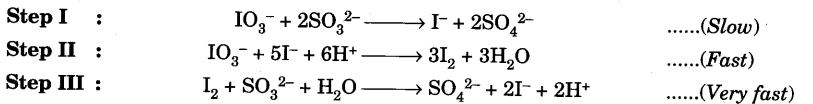
Sulphite ions react with potassium iodate producing iodide ions. Iodide ions, thus formed, are oxidized to iodine by reaction with more iodate ions. Iodine formed in Step II reacts immediately with sulphite ions forming iodide ions. When sulphite ions are completely consumed,the liberated iodine will not be consumed and would give blue colour, if starch is present. Thus, the above reaction can be monitored by adding a known but limited volume of sodium sulphite solution and starch solution. This is an example of clock reaction as the rate of the reaction is estimated by the time taken for the appearance of blue colour.
Apparatus and Chemicals
4 Conical flasks (250 ml), measuring cylinder, burette, pipette (25 ml), stop-watch.
0.01 M sodium sulphite solution, 0.1 M potassium iodate solution, starch solution, 2 M H2SO4.
Procedure
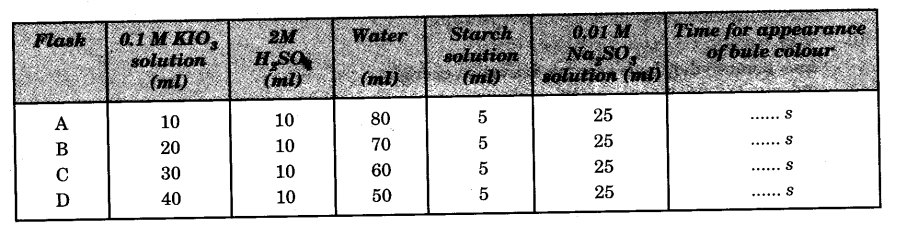
- Take four 250 ml conical flasks and label them as A, B, C and D.
- Add 10 ml, 20 ml, 30 ml and 40 ml of 0.1 M KIO3 solution to the flasks A, B, C and D respectively with the help of burette.
- Add 10 ml of 2 M H2SO4 to each flask.
- Add water to make the volume of solution 100 ml in each flask.
- Add 5 ml of freshly prepared starch solution to each flask.
- Add 25 ml of 0.01 M sodium sulphite solution to flask A with the help of a pipette and start the stop-watch immediately. Note the time when the blue colour just appears.
- Repeat the step 6 with the solutions of flasks B, C and D.
Observations
Conclusion
The rate of reaction increases with the increase in concentration of potassium iodate.
Precautions
- Always use a freshly prepared solution of sodium sulphide because it is easily oxidized by air.
- Concentration of KIO3 solution should be higher than the concentration of sodium sulphilic solution.
- Use a freshly prepared starch solution.