To Study the Effect of Change in Temperature on the Rate of Reaction Between Sodium Thiosulphate and Hydrochloric Acid
Chemistry Lab ManualNCERT Solutions Class 12 Chemistry Sample Papers
Theory
The rate of a chemical reaction depends to a great extent upon temperature. The rate of reaction increases with increase in temperature. Increase in temperature increases kinetic energy of the molecules. Therefore, the fraction of molecules having energy greater than its threshold energy increases which results in the increase in number of effective collisions per second. It has been observed that in most of the cases for every 10°C rise in temperature, the rate of the reaction becomes almost double. The rate of reaction between sodium thiosulphate and hydrochloric acid also increases with increase in temperature.
Apparatus
Conical flask (250 ml), measuring cylinders (50 ml and 5 ml), stop-watch, thermometer, tripod stand, wire-gauze and burner.
Materials Required
0.1 M Na2S203 solution, 1 M HCl, distilled water and cone. HN03.
Procedure
- Take 50 ml of 0.1 M Na2S203 solution in a 100 ml conical flask and note its temperature with the help of a thermometer.
- Add 10 ml of 1 M HCl to it and start the stop-watch immediately when half of the hydrochloric acid solution has been added.
- Shake the contents of the flask gently and place it on the tile with a cross-mark as shown in Fig.
- Observe the cross-mark from the top and note the time taken for the mark to become just invisible.
- Empty the flask and clean it thoroughly with cone. HN03 and then with water.
- Take again 50 ml of 0.1 M Na2S203 in conical flask and heat it so that the temperature of the solution becomes (T + 10°)C.
- Remove the flask from the tripod-stand and add 10 ml of 1 M HCl to it and start the stop-watch.
- Shake the contents gently and place it on the tile having a cross-mark.
- Note the time taken for the mark to become just invisible.
- Repeat the experiment at (T + 20)°C, (T + 30)°C and (T + 40)°C temperatures and record the observations as given below.
Observations
Volume of 0.1 M Na2S203 solution taken each time = 50 ml
Volume of 1 M HCl added each time = 10 ml.
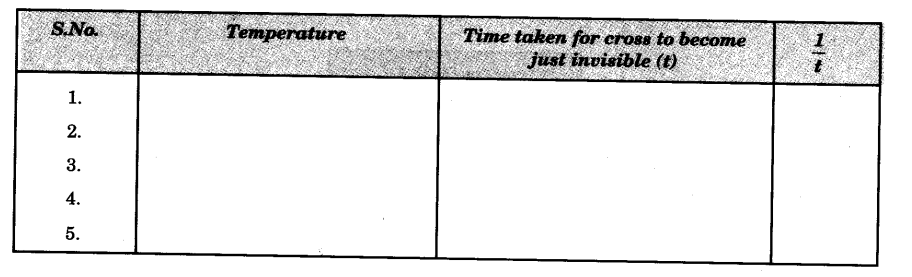
Plotting of Graph
Plot a graph by taking 1/t along the ordinate (vertical axis) and temperature along the abscissa (horizontal axis).
Result
Rate of reaction between sodium thiosulphate and hydrochloric acid increases with the increase in temperature.
Precautions
- The apparatus must be thoroughly clean. If the same conical flask is to be used again and again, it should be thoroughly washed with cone. HN03 and then with water.
- Measure the volumes of sodium thiosulphate solution, hydrochloric acid and distilled water very accurately.
- Use the same tile with the same cross-mark for all observation,
- Complete the experiment at one time only so that there is not much temperature variation.
- Start the stop-watch immediately when half of the hydrochloric acid solution has been added to sodium thiosulphate solution.
- View the cross-mark through the reaction mixture from top to bottom from same height for all observations.
<!–
–>