To Set up Simple Daniell Cell Using Salt Bridge and Determine its emf
Theory
When a copper electrode dipped in copper sulphate solution is connected to a zinc electrode dipped in the zinc sulphate solution, then electrons flow from zinc electrode to copper electrode and the chemical reactions take place as :
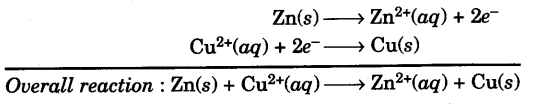
Apparatus and Chemicals
One beaker, a porous pot, connecting wires, milli voltmeter, sand paper, zinc strip, copper strip, 1 M ZnS04 solution and 1 M CuS04 solution.
Procedure
Set up the apparatus as shown in Fig.
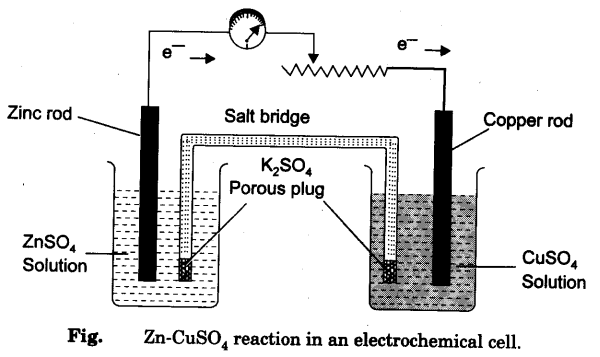
containing 1 M solutions of ZnS04 and CuS04 and note the position of the pointer in the voltmeter and record the reading in your record book.
Note : 1. Use of salt bridge gives a more efficient cell as it even prevents the diffusion of solvent molecules resulting from concentration difference.
2. Electrolytes in salt bridge can also be potassium chloride or potassium nitrate containing agar- agar (3 g in 100 ml of the saturated solution).
Observation
The emf of the Daniell cell is volts.
Precautions
- The concentration of copper sulphate and zinc sulphate should neither be too low nor too high.
- The porous pot should not be completely dipped into the copper sulphate solution, i.e., the copper sulphate solution should not be allowed to enter into the porous pot.
- Clean zinc and copper strips with sand paper before use.
- Carry out dilution of the solution carefully.
- Note the reading only when the pointer becomes stable.
- Connect copper strip with the positive terminal of voltmeter and zinc strip with negative terminal.
Chemistry Lab ManualNCERT Solutions Class 12 Chemistry Sample Papers
<!–
–>