Prepare M/20 Solution of Ferrous Ammonium Sulphate (Mohr’s salt). Using this Solution Find out the Molarity and Strength of the Given KMnO4 Solution
Chemical Equations
Indicator
KMnO4 is a self-indicator.
End Point
Colourless to permanent pink colour (KMnO4 in burette).
Procedure
- Prepare 250 ml of M/20 Mohr’s salt solution by dissolving 4.9 g of Mohr’s salt in water 20 as described in experiment 11.3. Rinse the pipette with the M/20 Mohr’s salt solution and pipette out 20.0 ml of it in a washed titration flask.
- Rinse and fill the burette with the given KMn04 solution.
- Add one test-tube (~ 20 ml) full of dilute sulphuric acid (~ 2 M) to the solution in titration flask.
- Note the initial reading of the burette.
- Now add KMnO4 solution from the burette till a permanent light pink colour is imparted to the solution in the titration flask on addition of last single drop of KMnO4 solution.
- Note the final reading of the burette.
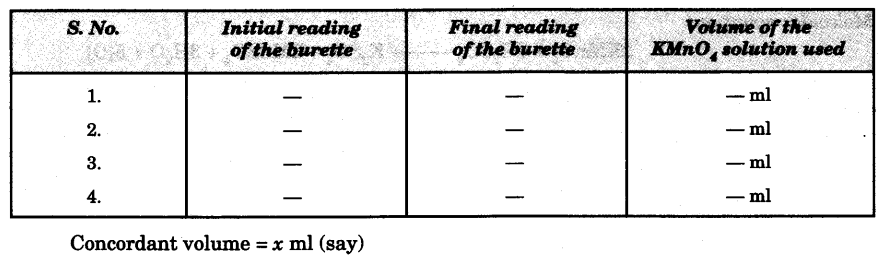
- Repeat the above steps 4-5 times to get a set of three concordant readings.
Observations
Weight of watch glass =……. g
Weight of watch glass + Mohr’s salt =…………..g
Weight of Mohr’s salt = 4.90 g
Volume of Mohr’s salt solution prepared = 250 ml
Molarity of Mohr’s salt solution = M/20
Volume of Mohr’s salt solution taken for each titration = 20.0 ml
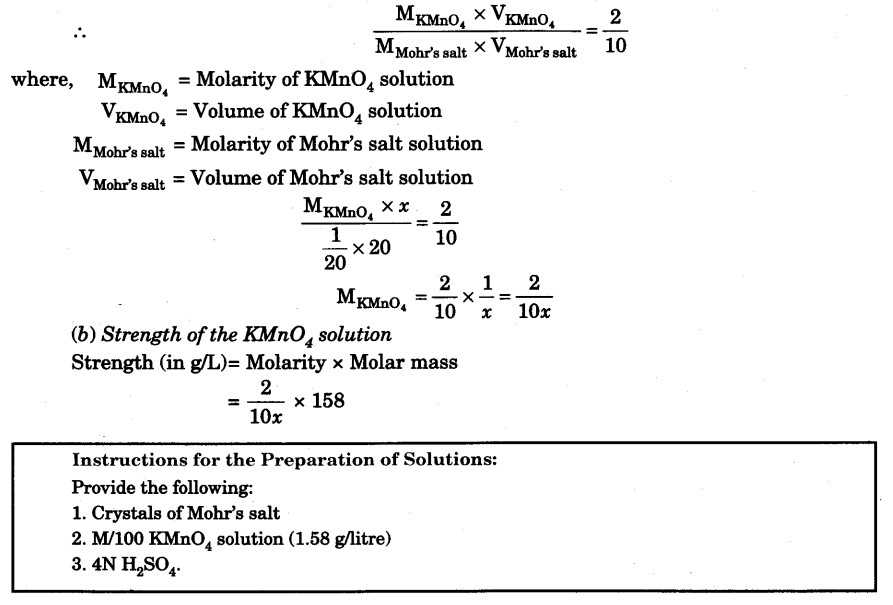
Calculations
(a) Molarity of the KMnO4 solution.
From the overall balanced chemical equation, it is clear that 2 moles of KMnO4 reacts with 10 moles of Mohr’s salt.
Chemistry Lab ManualNCERT Solutions Class 12 Chemistry Sample Papers
<!–
–>