Find out the Percentage Purity of Impure Sample of Oxalic Acid.You are Supplied M/100 KMnO4 Solution
Chemistry Lab ManualNCERT Solutions Class 12 Chemistry Sample Papers
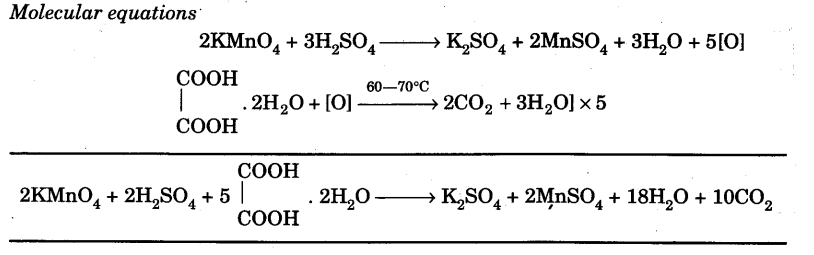
Chemical Equations
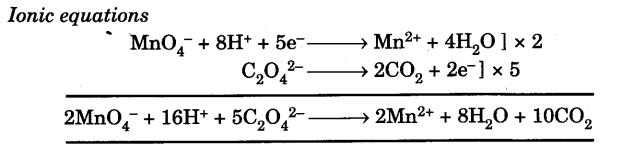
Indicator
KMnO4 is a self-indicator.
End Point
Colourless to permanent pink colour (KMnO4 in burette).
Procedure
- Weigh exactly 2.0 g of oxalic acid and dissolve in water to prepare 500 ml of its solution using a 500 ml measuring flask. Rinse the pipette with the oxalic acid solution and pipette out 20 ml of it in a washed titration flask.
- Rinse and fill the burette with M/100 KMnO4 solution.
- Add one test-tube (~ 20 ml) full of dilute sulphuric acid (~ 2 M) to the solution in titration flask.
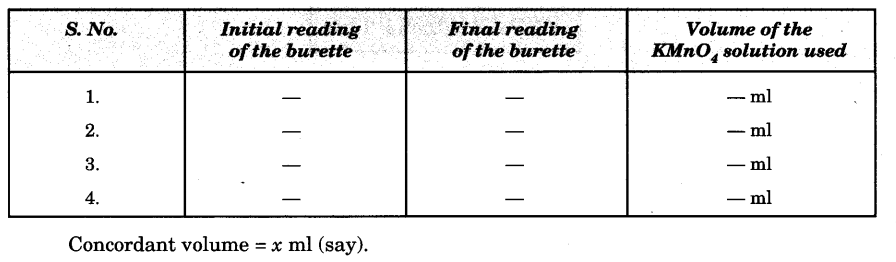
- Note the initial reading of the burette.
- Heat the flask to 60-70°C and add KMnO4 solution from the burette till a permanent light pink colour just appears in the solution in the titration flask.
- Note the final reading of the burette.
- Repeat the above steps 4-5 times to get a set of three concordant readings.
Observations
Weight of watch glass =……. g
Weight of watch glass + Mohr’s salt =…………..g
Weight of Mohr’s salt = 2.00 g
Volume of Mohr’s salt solution prepared = 500 ml
Solution taken in burette = M/100 KMnO4
Volume of Mohr’s salt solution taken for each titration = 20.0 ml
Calculations
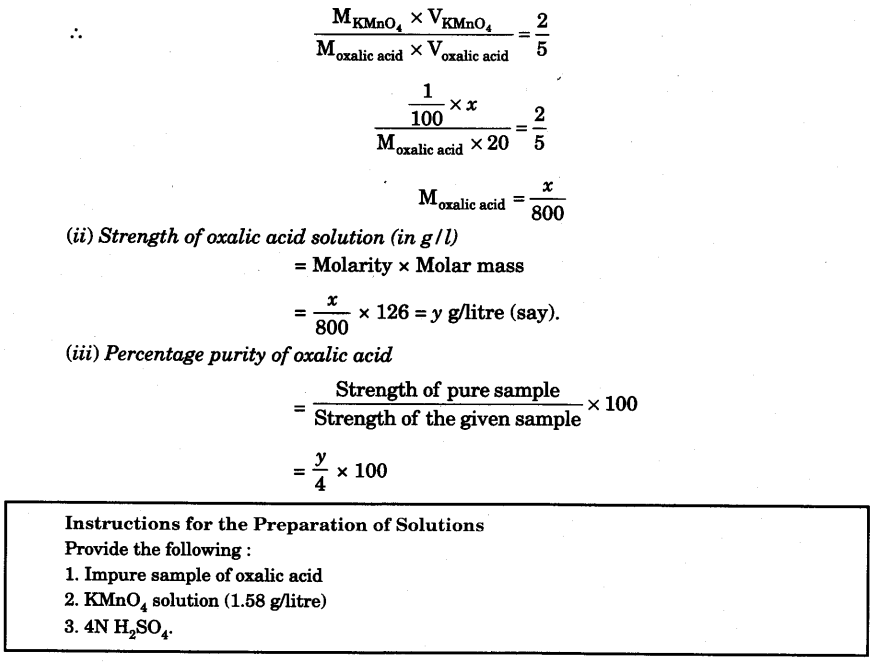
(a) Molarity of the KMnO4 solution
From the overall balanced chemical equation it is clear that 2 moles of KMnO4 react with 5 moles of oxalic acid.
<!–
–>