Prepare (frac{M}{10}) sodium carbonate solution
Chemistry Lab ManualNCERT Solutions Class 11 Chemistry Sample Papers
Theory
Sodium carbonate is a primary standard. Its molecular mass is 106.
To prepare (frac{M}{10}) Na2CO3 solution, 10.6 g of sodium carbonate should be dissolved per litre of the solution. Normally in the laboratory we are required to prepare 250 ml of solution. Therefore, to prepare 250 ml of (frac{M}{10}) Na2CO3 solution, (frac{10.6}{4}) = 2.650 g of sodium carbonate are dissolved in lesser quantity of water and the solution diluted to exactly 250 ml.
Apparatus
Weight box, chemical balance, watch glass, 250 ml beaker, glass rod, 250 ml measuring flask, wash bottle.
Procedure
1. Take a watch glass, wash it with distilled water and then dry it.
2. Weigh the clean and dried watch glass accurately and record its weight in the note-book.
3. Weigh 2.650 g sodium carbonate on the watch glass accurately and record this weight in the note-book.
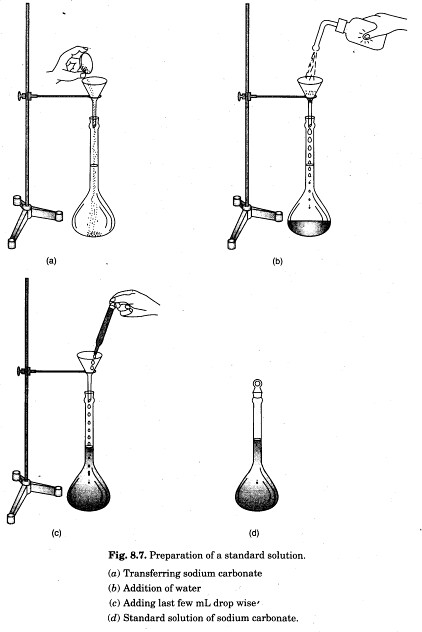
4. Transfer gently and carefully sodium carbonate from the watch glass into a clean and dry measuring flask using a funnel. Wash the watch glass with distilled water with the help of a wash bottle to transfer the particles sticking to it into funnel [Fig. 8.7(a)]. The volume of distilled water for this purpose should not be more than 50 ml.
5. Wash funnel several times with distilled water by using a wash bottle to transfer the sticking particles into the measuring particles into the measuring flask. While washing the funnel, add water in small amounts. The volume of distilled water used for this purpose should not be more than 50 mL.
6. Finally wash the funnel thoroughly with distilled water with the help of a wash bottle to transfer the solution sticking to the funnel into the measuring flask [Fig. 8.7(6)].
7. Swirl the measuring flask till solid sodium carbonate dissolves.
8. Add enough distilled water to the measuring flask carefully upto just below the etched mark on it, with the help of wash bottle.
9. Add the last few mL of distilled water dropurse until the lower level of the meniscus just touches the mark on the measuring flask [Fig. 8.7(c)].
10. Stopper the measuring flask and shake gently to make the solution uniform throughout. Label it as (frac{M}{10}) sodium carbonate solution.
<!–
–>