Experiments Based On pH Change
pH SCALE
In order to express the hydronium ion (H3O+) concentration in a solution P.L. Sorensen (1909) devised a logarithmic scale. This scale is known as pH scale. The pH of a solution is defined as the negative logarithm of hydronium ion concentration in moles per litre.
(pH=-log { { [H }_{ 3 } } { O }^{ + }])
= (logfrac { 1 }{ { H }_{ 3 }{ O }^{ + } } )
Acidity, Alkalinity, Neutrality of Solutions
neutral solution : ({ H }^{ + }) = ({OH}^{-}) = ({10}^{-7})M ; pH = – 7
acidic solution : ({ H }^{ + }) > ({OH}^{-}), ({ H }^{ + }) > ({10}^{-7})M, pH < 7.
basic solution: ({OH}^{-}) > ({ H }^{ + }), ({ H }^{ + }) < ({10}^{-7})M, pH > 7. (also called an alkaline solution)
Strong and Weak Acids and Bases
strong acid—an acid that is a strong electrolyte and has a pH < 3.
For example, (H_{2}SO_{4}), (HCl), (HBr), (HI), (HNO_{3})
weak acid—an acid that is a weak electrolyte or an ionic compound that partially reacts with water to form hydrogen ions in aqueous solution. It will have a pH greater than 3 but less than 7.
For example, (H_{2}S), (H_{3}PO_{4}), (CH_{3}COOH), (H_{2}CO_{3}).
strong base—a hydroxide that is a strong electrolyte and has a pH >11.
For example, (NaOH), (KOH), (Ba(OH)_{2}).
weak base—a hydroxide that is a weak electrolyte or a compound that partially reacts with water to form hydroxide ions in aqueous solution. Its pH will be less than 11 but greater than 7.
For example, carbonates, bicarbonates, ammonia (ammonium hydroxide), phosphates.
salt—an ionic compound produced by reacting an acid and a base. It will have a pH close to 7.
Ioconic Product Of Water
Pure water is very weakly ionised. So there is an equilibrium between ionised and unionised molecules.
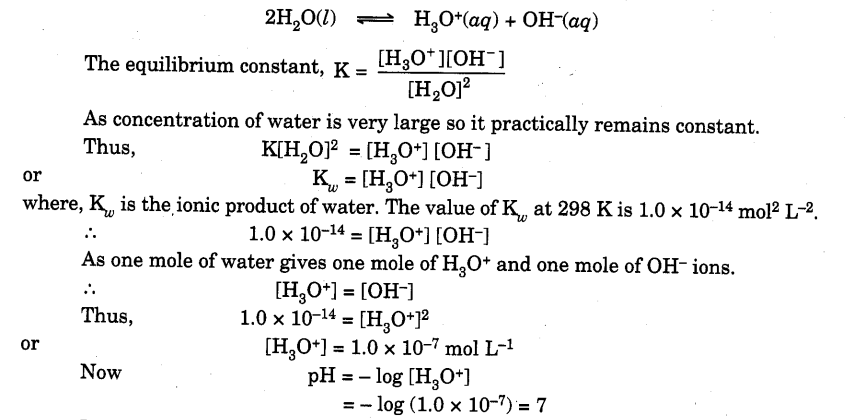
In general, it has been observed that at room temperature all neutral solutions have pH equal to 7, all acidic solutions have pH less than 7 and all basic solutions have pH more than 7.
Common Ion Effect
Common ion effect may be defined as the supression of degree of dissociation of a weak electrolyte by the addition of a small amount of some strong electrolyte having a common ion with that of the weak electrolyte.
Consider for example, (NH_{4}OH) which is a weak electrolyte and there is an equilibrium between unionised molecules and its ions.
![]()
When (NH_{4}Cl), a strong electrolyte, is added to it, (NH_{4}Cl) ionises as
![]()
Due to the presence of common (NH_{4}^+) ions the equilibrium (6.1) shifts in the backward direction and degree of dissociation of (NH_{4}OH) is supressed. So the concentration of ({OH}^{-}) ions decreases and hence concentration of (H_{3}O^+) ions increases. Thus, pH of the solution is lowered.
Similarly consider acetic acid, a weak electrolyte
![]()
When sodium acetate, a strong electrolyte is added to it, CHgCOONa ionises as :
![]()
Due to the presence of common (CH_{3}COO^{-}) ions the equilibrium (6.2) shifts in the backward direction and so concentration of (H_{3}O^+) ions decreases and hence that of OH- ions in¬creases. Therefore, pH of solution increases.
Salts when dissolved in water may undergo hydrolysis producing acidic or basic solutions. Hydrolysis of salts may be defined as the interaction of ions of the salt with water producing acidic or basic solution.
Hydrolysis of salts of strong bases and weak acids produces alkaline solution on hydrolysis. For example, the aqueous solution of sodium acetate is alkaline due to the presence of excess hydroxyl ions in the solution.
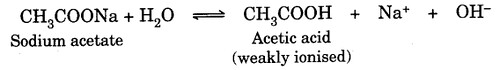
Hydrolysis of salts of strong acids and weak bases produces acidic solution due to the presence of excess hydronium ions in the solution. For example, an aqueous solution of ammonium chloride is acidic in nature.
![]()
Hydrolysis of salts of weak acids and weak bases gives almost neutral solutions. For example,
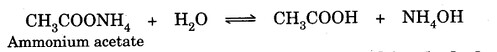
Salts of strong acids and strong bases donot undergo hydrolysis and hence their aqueous solutions are neutral.
Table 6.1. Colour Changes and pH range of Certain Indicators
| S.No. | Indicator | pH range | Colour in acidic medium | Colour in alkaline medium |
| 1. | Thymol blue | 1.2-2.8 | Red | Yellow |
| 2. | Methyl yellow | 2.9-4.0 | Red | Yellow |
| 3. | Bromophenol blue | 3.0-4.6 | Yellow | Blue |
| 4. | Congo red | 3.0-5.0 | Violet | Red |
| 5. | Methyl orange | 3.1-4.4 | Red | Yellow |
| 6. | Methyl red | 4.2-6.3 | Red | Yellow |
| 7. | Phenol red | 6.8-8.4 | Yellow | Red |
| 8. | Phenolphthalein | 8.3-10.0 | Colourless | Pink |
| 9. | Thymolphthalein | 9.4-10.5 | Colourless | Blue |
Universal Indicator
A universal indicator is prepared by mixing a number of common indicators together so that the mixture obtained can pass through a series of colour changes over a much wider pH range. For example, one such mixture which may show various colours at different pH is as given in Table 6.2.
Table 6.2. Colours of Universal Indicator at Different pH Values
| pH | Colour |
| 3.0 | Red |
| 5.0 | Orange red |
| 5.5 | Orange |
| 6.0 | Orange-yellow |
| 7.0-7.5 | Greenish-yellow |
| 8.0 | Green |
| 9.5 | Blue |
| 10.0 | Violet |
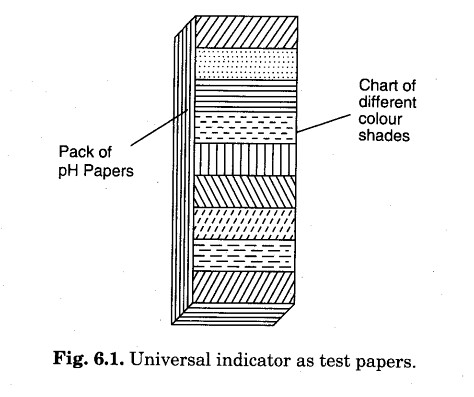
Such mixtures are commonly known as universal indicators. Universal indicators are available commercially as solutions and as test papers. A pH paper is a strip of paper which is prepared by dipping the strip in the solutions of different indicators and then drying them.
pH paper can be used to find the approximate pH of any solution. The pH paper is dipped in a given sample of the solution, the colour developed in the paper is compared with the colour chart and approximate pH of the solution can be predicted. A pH paper is shown in Fig. 6.1.
Chemistry Lab ManualNCERT Solutions Class 11 Chemistry Sample Papers
<!–
–>